Note 1
Isotopes of elements are normally identified using this notation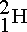 This example considers the isotope of Hydrogen, H is the chemical symbol for Hydrogen, 1 is the atomic number (number of protons) and 2 is the atomic mass (protons + neutrons). Unfortunately,
the limitations of some web browsers make it impracticable for me to follow the normal notation within this page. For this reason
I will be using the ammended notation of 21H
This example considers the isotope of Hydrogen, H is the chemical symbol for Hydrogen, 1 is the atomic number (number of protons) and 2 is the atomic mass (protons + neutrons). Unfortunately,
the limitations of some web browsers make it impracticable for me to follow the normal notation within this page. For this reason
I will be using the ammended notation of 21HNote 2
These series are the ones regularly quoted in text books, however, there are many other short series (often only involving a single decay before becoming stable,) there could be additional long series and it is possible to extend these series to encompass many of the synthetic elements generated artificially.Thorium Series
| Element | Decay | Half Life |
|---|---|---|
| 25298Cf | Alpha 42α | 2.6 years |
| 24896Cm | Alpha 42α | 3.4 x105 years |
| 24494Pu | Alpha 42α | 8.0 x107 years |
| 24092U | Beta 0-1β | 14.1 hours |
| 24093Np | Beta 0-1β | 1.0 Hours |
| 24094Pu | Alpha 42α | 6.5 x103 years |
| 23692U | Alpha 42α | 2.3 x107 years |
| 23290Th | Alpha 42α | 1.4 x1010 Years |
| 22888Ra | Beta 0-1β | 6.7 Years |
| 22889Ac | Beta 0-1β | 6.1 Hours |
| 22890Th | Alpha 42α | 1.9 Years |
| 22488Ra | Alpha 42α | 3.6 Days |
| 22086Rn | Alpha 42α | 55 Seconds |
| 21684Po | Alpha 42α | 3.0 x10-7 Seconds |
| 22282Pb | Beta 0-1β | 10.6 Hours |
| 21283Bi | Alpha 42α Beta 0-1β | 3.0 x10-7 Seconds |
| If decay of 21283Bi is α | ||
| 20881Tl | Beta 0-1β | 3.1 Minutes |
| 20882Pb | Stable | |
| If decay of 21283Bi is β | ||
| 21284Po | Alpha 42α | 25 Seconds |
| 20882Pb | Stable | |
Uranium Series (Radium Series)
| Element | Decay | Half Life |
|---|---|---|
| 23892U | Alpha 42α | 4.5 x109 Years |
| 23490Th | Beta 0-1β | 2.4 Days |
| 23491Pa | Beta 0-1β | 6.7 Hours |
| 23492U | Alpha 42α | 2.4 x105 Years |
| 23090Th | Alpha 42α | 8 x104 Years |
| 22688Ra | Alpha 42α | 1.6 x103 Years |
| 22286Rn | Alpha 42α | 3.8 Days |
| 21884Po | Alpha 42α | 3.1 Minutes |
| 21482Pb | Beta 0-1β | 27 Minutes |
| 21483Bi | Alpha 42α Beta 0-1β | 20 Minutes |
| If decay of 21483Bi is α | ||
| 21081Tl | Beta 0-1β | 1.8 Minutes |
| 21082Pb | Beta 0-1β | 20 Years |
| 21083Bi | Alpha 42α | 5 Days |
| 20681Tl | Beta 0-1β | 24.2 Minutes |
| 20682Pb | Stable | |
| If decay of 21483Bi is β | ||
| 21484Po | Alpha 42α | 1.6 x10-4 Seconds |
| 21082Pb | Beta 0-1β | 20 Years |
| 21083Bi | Alpha 42α | 5 Days |
| 20681Tl | Beta 0-1β | 24.2 Minutes |
| 20682Pb | Stable | |
Actinium Series
| Element | Decay | Half Life |
|---|---|---|
| 23994Pu | Alpha 42α | 2.4 x104 Years |
| 23592U | Alpha 42α | 7.1 x108 Years |
| 23190Th | Beta 0-1β | 26 Hours |
| 23191Pa | Alpha 42α | 3.2 x104 Years |
| 22789Ac | Beta 0-1β | 22 Years |
| 22790Th | Alpha 42α | 18 Days |
| 22388Ra | Alpha 42α | 11 Days |
| 21986Th | Alpha 42α | 4 Seconds |
| 22584Po | Alpha 42α | 1.8 x10-3 Seconds |
| 21182Pb | Beta 0-1β | 36 Minutes |
| 21183Bi | Alpha 42α | 2.2 Minutes |
| 20781Tl | Beta 0-1β | 4.8 Minutes |
| 20782Pb | Stable |
Neptunium Series
| Element | Decay | Half Life |
|---|---|---|
| 24998Cf | Alpha 42α | 351 years |
| 24596Cm | Alpha 42α | 8.5 x103 years |
| 24194Pu | Beta 0-1β | 14.4 years |
| 24195Am | Alpha 42α | 432 years |
| 23793Np | Alpha 42α | 2.1x 106 years |
| 23391Pa | Beta 0-1β | 27 days |
| 23392U | Alpha 42α | 1.6x 105 years |
| 22990Th | Alpha 42α | 7.3 x103 years |
| 22588Ra | Beta 0-1β | 14.9 days |
| 22589Ac | Alpha 42α | 10 days |
| 22187Fr | Alpha 42α | 4.8 minutes |
| 21785At | Alpha 42α | 32x 10-3 seconds |
| 21383Bi | Alpha 42α Beta 0-1β | 46.5 minutes |
| If decay of 21383Bi is α | ||
| 20981Tl | Beta 0-1β | 2.2 min |
| 20982Pb | Beta 0-1β | 3.2 hours |
| 20983Bi | Alpha 42α | 1.9x 1019 years |
| 20581Tl | stable | |
| If decay of 21383Bi is β | ||
| 21384Po | Alpha 42α | 3.7x 10-6 seconds |
| 20982Pb | Beta 0-1β | 2.2 min |
| 20983Bi | Alpha 42α | 1.9x 1019 years |
| 20581Tl | stable | |
www.DigitalDan.co.uk